The Application and Significance of Zeta Potential Measurement to Study Bacterial Interaction With Foods
By J. Li & L. A. McLandsborough
Department of Food Science, University of Massachusetts, Amherst
Our laboratory is studying the adhesion of bacteria to foods using E. coli as our model organism. We have been using zeta potential measurement to evaluate the electrostatic interaction between bacterial cells and food emulsions.
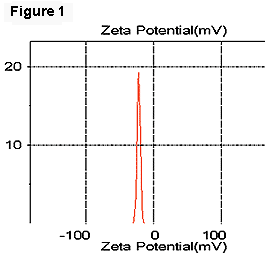
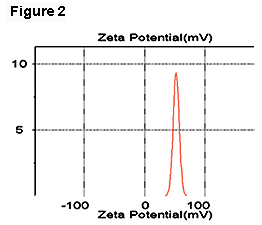
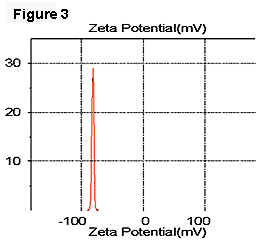
In our experiments, we used a laboratory strain of E. coli JM109 that has a zeta potential of -22 mV when suspended in PBS (Figure 1). The oil-in-water emulsions (hexadecane to water, 40% by volume) were prepared with a sonicator by adding 2% of dodecyl trimethylammonium bromide (DTAB) or sodium dodecyl sulfate (SDS) as the emulsifiers, resulting in zeta potentials of emulsion droplets of +52 mV and -82 mV, respectively (Figures 2 and 3).
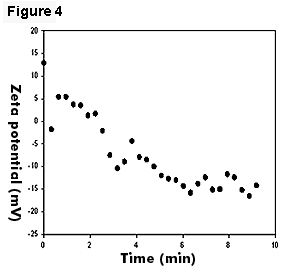
When bacterial cells were added to DTAB emulsions, the kinetics of interaction at the early stage was studied with sequential measurements. Only one population of droplets was found and this may indicate the binding of cells to emulsion droplets. We were able to observe a rapid change in charge from +15 mV to -15 mV over a course of about 6 minutes, at which time the charge was stabilized at around -15 mV (Figure 4). We believe that Figure 6 illustrates the rate of interaction between the negatively charged bacterial cells and the positively charged DTAB emulsion droplets.
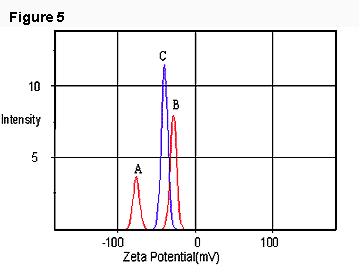
With the same technique, two situations were observed for the mixture of bacterial cells and SDS emulsions. In one case, we found two populations of droplet zeta potential (Figure 5): peak A (close to SDS emulsion droplets) and peak B (close to bacterial cells). In the other case, only one population (peak C) could be detected (Figure 5), which was apparently the result of the interaction between cells and the emulsion droplets and may be due to forces other than electrostatic attraction.
The interaction and association of bacterial cells and the emulsion droplets was confirmed by fluorescent microscopic analysis.
Zeta potential measurement is a fast and reliable evaluation of bacterial surface charge properties. Kinetic study of the interaction between bacterial cells and various emulsion droplets provides an excellent model for the investigation of bacterial interaction with foods.
Acknowledgment
This research was supported by USDA NRI grant #97-35201-4508. We thank Dr. D. Julian McClements for technical assistance with zeta potential measurements and with emulsion.
References
James, A.M., Charge properties of microbial cell surfaces, in Microbial cell surface analysis-structural and physicochemical methods, N. Mozes, P. S. Handley, H. J. Busscher, P. G. Rouxhet, Editors. 1991, VCH Publishers, Inc.: New York. p. 221-262.
Dr. Jack Li
Phone: (413) 545-1024
Email: jkli@foodsci.umass.edu
Dr. L. A. McLandsborough
University of Massachusetts
Department of Food Science
Chenoweth Laboratory
Amherst, MA 01003
Phone : (413) 545-1016; Fax: (413) 545-1262
Email: lm@foodsci.umass.edu
This article is reprinted from Malvern Instruments' Particle Techniques newsletter.
